| 品牌 |
货号 |
纯度标准 |
包装 |
价格/优惠价 |
折扣价 |
特价 |
库存 |
数量1 |
购买 |
| J&K |
A01_292464 |
98% |
25g |
30.00/0 |
- |
- |
查看 |
|

|
| J&K |
A01_292464 |
98% |
100g |
119.00/0 |
- |
- |
查看 |
|

|
| J&K |
A01_292464 |
98% |
500G |
476.00/0 |
- |
- |
查看 |
|

|
| J&K |
A01_292464 |
98% |
1KG |
893.00/0 |
- |
- |
查看 |
|

|
| J&K |
A01_292464 |
98% |
2.5KG |
2142.00/0 |
- |
- |
查看 |
|

|
|
|
产品信息安全信息参考文献
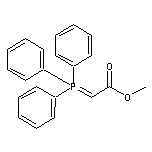
Stable ylide which undergoes the Wittig reaction with aldehydes to give substituted methyl acrylates. For a detailed study of the reaction with benzaldehyde, see J. Org. Chem., 59, 1126 (1994). For use of high pressure to increase the yield and trans-selectivity of reaction with aromatic aldehydes, see: Liebigs Ann. Chem., 2135 (1983). For tandem Wittig reaction and Cope rearrangement, see: J. Chem. Soc., Chem. Commun., 381 (1995).Strong base, for example Na bis(TMS)amide, brings about elimination of methanol, giving ketenylidenetriphenylphosphorane, which reacts with ɑ-hydroxy-ketones, e.g. in the steroid series, to give butenolides, by acylation and Wittig cyclization: Chem. Ber., 113, 2038 (1980). Review of phosphacumulene ylides: Angew. Chem. Int. Ed., 16, 349 (1977).